A new drug that starves cancer cells of their energy source has shown promising results in a clinical trial for patients with mesothelioma, a rare and incurable cancer caused by exposure to asbestos. The drug, called ADI-PEG20, was combined with chemotherapy and increased the median survival by 1.6 months and the three-year survival by four times, compared to chemotherapy alone.
The study, published in JAMA Oncology, was led by researchers from Queen Mary University of London and The University of Western Australia, and involved 249 patients from five countries: the UK, US, Australia, Italy and Taiwan. The trial ran from 2017 to 2021 and was the first to test the efficacy and safety of ADI-PEG20 for mesothelioma.
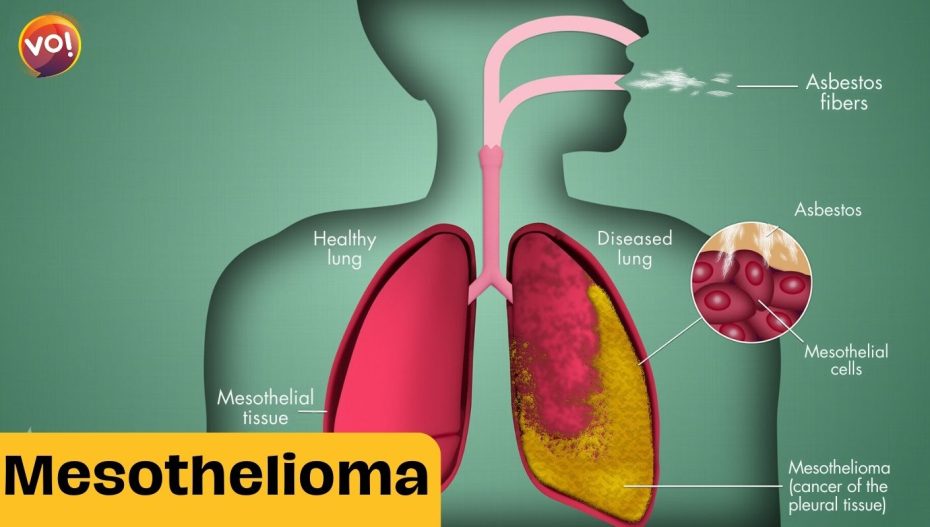
Mesothelioma affects the lining of the lungs, abdomen, heart or testicles, and is usually diagnosed at an advanced stage. It is often linked to occupational exposure to asbestos, a fibrous mineral that was widely used in construction and insulation materials until it was banned in many countries. About 2,500 people are diagnosed with mesothelioma in the UK each year, and the disease has a poor prognosis, with a median survival of less than a year.
ADI-PEG20 works by depleting the levels of arginine, an amino acid that is essential for the growth and survival of mesothelioma cells, but not normal cells. By depriving the cancer cells of arginine, the drug triggers a process called autophagy, in which the cells start to consume themselves. This makes the cancer cells more vulnerable to chemotherapy, which can then kill them more effectively.
The researchers, including Prof Peter Szlosarek from Queen Mary who led the study, wrote: “In this pivotal, randomised, placebo-controlled, phase 3 trial in 249 patients with pleural mesothelioma, pegargiminase-chemotherapy increased significantly the median overall survival by 1.6 months and quadrupled the survival at 36 months compared to placebo-chemotherapy. Pegargiminase-based chemotherapy was well tolerated with no new safety signals.”
Dr Jyoti Mehta, MD Radiation and Clinical Oncologist at TGH Onco Life Cancer Centre in Talegaon, India, who was not involved in the study, praised the findings in an interview with HT Lifestyle. She said: “The success of this clinical trial raises optimism within the scientific community, emphasizing the importance of collaborative research and the pursuit of novel treatment strategies. The integration of ADI-PEG20 with chemotherapy offers a beacon of hope for patients battling malignant mesothelioma, showcasing the potential for improved outcomes and an enhanced quality of life.”
She added: “While further studies and clinical validations will be crucial, these early findings underscore the importance of continued investment in research and development to bring forth transformative solutions for challenging medical conditions. The scientific community eagerly awaits more detailed insights into the mechanisms and long-term impacts of ADI-PEG20, with the hope that it may emerge as a truly wonderful advancement in the treatment landscape for asbestos-linked cancers.”
Oat Lovers Beware: Study Finds ‘Fertility Impairing’ Pesticide In 80% of Americans. Click Here