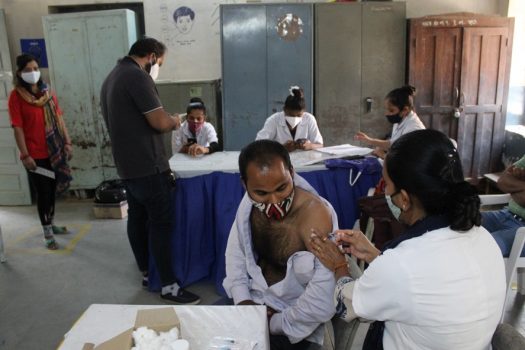
India: Corbevax, Covaxin And Zycov-D Get Emergency Use Permit
April 26, 2022 15:51The Indian drug regulator has granted emergency use authorization for Biological E’s COVID-19 vaccine Corbevax for children aged five to twelve years, ZycovD (Zydus Cadila vaccine) for children aged twelve and up, and Bharat Biotech’s Covaxin for children aged six to twelve years. The approval by the Drugs Controller General of India (DCGI) follows recommendations […]